From his fifth-grade class in Huntington, Indiana, through his zoology major at DePauw University to his lab at Indiana University School of Medicine, Mark Kelley ’79 has followed the science.
“It’s always about following the protein, following what it does, and then following the science,” he said. And for almost 30 years, Kelley has followed a protein called APE1/Ref-1.
“The story gets very complicated,” he said. “It’s really hard to do an elevator pitch.” But Kelley’s work comes down to this: Promising treatments for pediatric and adult brain tumors; pediatric leukemia and neuroblastoma; osteosarcoma; and pancreatic, ovarian, bladder, prostate and cervical cancers. For diabetic retinopathy and diabetic macular edema. For inflammatory bowel disease and other diseases of the digestive tract. Maybe more.
“My lab and my partners’ labs are all about translation,” he said. “I know that’s a big buzzword, but we want to translate our findings from the bench to the public. That’s what our focus is. We like basic science but we always want to be thinking: Where is this going to go to help the patient? I love science for science, but I also love when I can see where this can have an impact.”
His grade school teacher may have sparked his interest in science, but independent research with fruit flies at DePauw and a study-away program at Oak Ridge National Laboratory caused him to realize his calling was research.
He worked with fruit flies throughout his master’s and Ph.D. programs and postdoctoral work at Rockefeller University, then went to work for Stritch School of Medicine at Loyola University, where “we started morphing from fruit fly research into mammalian cells – cell culture in mice and humans. That’s when I started working on this protein.”
“We always want to be thinking: Where is this going to go to help the patient? I love science for science, but I also love when I can see where this can have an impact.”– Mark Kelley '79
In 1993, he joined IU, where his various titles and appointments rivals those of Elizabeth II. For this story, his most pertinent roles are associate director of basic science research at IU’s Simon Cancer Center and the Betty and Earl Herr chair in pediatric oncology research.
Late last decade, Kelley, several other scientists and investors formed Apexian Pharmaceuticals Inc. to advance the clinical trials of his drugs. IU owns the intellectual property Kelley has developed, but licensed his patents to Apexian, which started a Phase 1 trial in February 2019 to determine the safety and dosing of his drug. The growth of tumors slowed in several participants, all of whom had end-stage cancer.
“We figured they’d be on a few cycles, we’d get our safety data and stop,” Kelley said. “But if patients’ tumors aren’t progressing, it’s just immoral and unethical to stop.”
The pharmaceutical world took notice of the drug’s safety profile and potential uses. In January, Apexian licensed potential drugs for the eye to Ocuphire Pharma Inc., which is expected to start a Phase II clinical trial, when efficacy will be evaluated, yet this year. Meanwhile, Apexian is getting inquiries about licensing its other pipeline drugs.
“You can’t imagine the feeling of taking something that you spend your life on in the lab, and you’re seeing a patient take this and you’re confident that it’s going to be safe,” Kelley said. “… It’s just fabulous, because that’s why we’re doing the science.”
Photo courtesy of Tim Yates, IU School of Medicine.
DePauw Magazine
Summer 2020
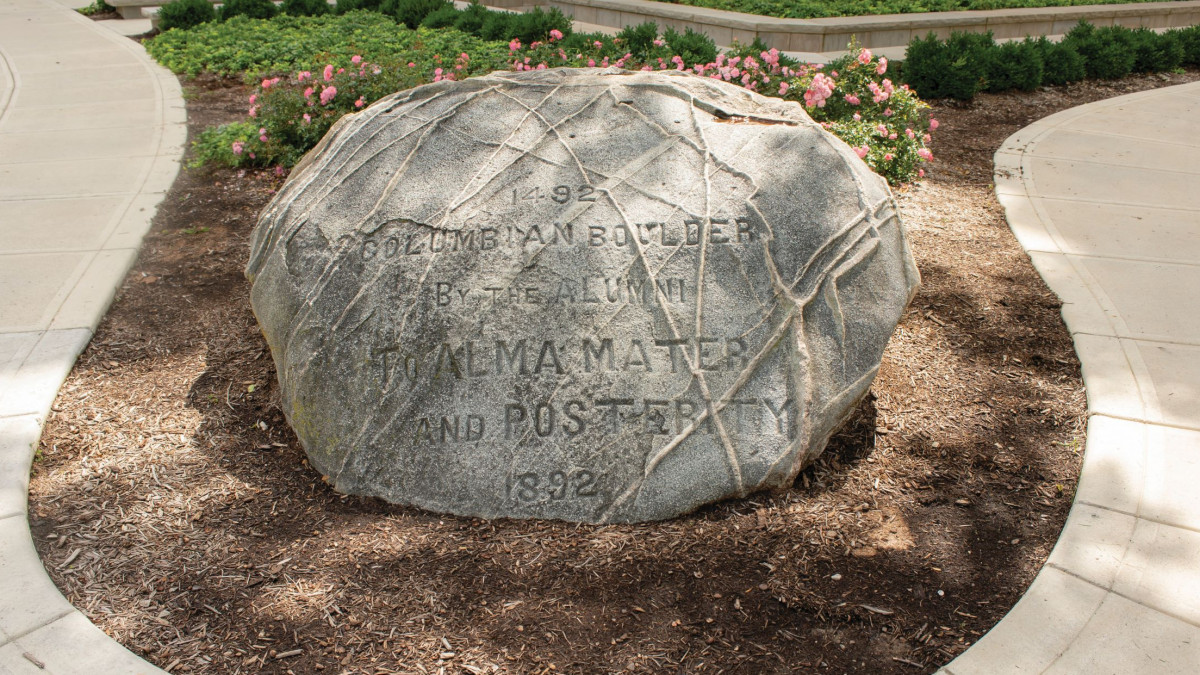 The Bo(u)lder Question
The Bo(u)lder Question  Retired archivist reflects on 36 years as DePauw’s memory keeper
Retired archivist reflects on 36 years as DePauw’s memory keeper First Person by Connie Campbell Berry '67
First Person by Connie Campbell Berry '67 First Person by Wayne Glausser
First Person by Wayne Glausser Battling an epidemic, treating individuals: Physician alum has done it all
Battling an epidemic, treating individuals: Physician alum has done it all  Welcome, Class of 2024!
Welcome, Class of 2024! She has loved them since she was 6: vet cares for, competes with and rescues horses
She has loved them since she was 6: vet cares for, competes with and rescues horses Liberal arts taught wildlife vet to consider different approaches to patients’ problems
Liberal arts taught wildlife vet to consider different approaches to patients’ problems Scientist and humanitarian: Prof embodies disparate interests, then acts on and teaches them
Scientist and humanitarian: Prof embodies disparate interests, then acts on and teaches them Researcher follows the science toward treatments
Researcher follows the science toward treatments The ‘dura mater’ handles medical training and motherhood with aplomb
The ‘dura mater’ handles medical training and motherhood with aplomb  Evolving interests drive ’12 grad to trade test tubes for a stethoscope
Evolving interests drive ’12 grad to trade test tubes for a stethoscope Alum hopes to meet global needs by establishing med school
Alum hopes to meet global needs by establishing med school The healers
The healers Personal experiences prepared ’76 alum for work, service
Personal experiences prepared ’76 alum for work, service DePauw in the time of COVID-19
DePauw in the time of COVID-19 DePauw’s new president: A ‘visionary,’ empathetic and focused optimist ... who sings
DePauw’s new president: A ‘visionary,’ empathetic and focused optimist ... who sings
DePauw Stories
A GATHERING PLACE FOR STORYTELLING ABOUT DEPAUW UNIVERSITY
Browse other stories
-
Athletics
-
Football - Robby Ballentine Selected Academic All-America® Team Member of the Year
-
Men's Basketball - DePauw Picks Up NCAC Road Win at Kenyon
-
Women's Basketball - Tigers Hang on for Win over Wooster
More Athletics
-
-
News
-
Student and Professor Share Unexpected Writing Journey
-
Four in a Row! DePauw Wins 131st Monon Bell Classic
-
Jim Rechtin '93 Featured in Fortune Magazine
More News
-
-
People & Profiles
-
Entrepreneurs Eric Fruth ’02 and Matt DeLeon ’02 Are Running More Than a Business
-
Rick Provine Leaves Legacy of Leadership and Creativity
-
History Graduate Cecilia Slane Featured in AHA's Perspectives on History
More People & Profiles
-
-
Have a story idea?
Whether we are writing about the intellectual challenge of our classrooms, a campus life that builds leadership, incredible faculty achievements or the seemingly endless stories of alumni success, we think DePauw has some fun stories to tell.
-
Communications & Marketing
101 E. Seminary St.
Greencastle, IN, 46135-0037
communicate@depauw.eduNews and Media
-
News media: For help with a story, contact:
Bob Weaver, Senior Director of Communications.
bobweaver@depauw.edu.